How Can You Remember the Sugar Sticks to Both Nucleotide Parts
Nucleic Acids
one. Introduction
The first isolation of what we now refer to as Dna was accomplished past Johann Friedrich Miescher circa 1870. He reported finding a weakly acidic substance of unknown function in the nuclei of human white blood cells, and named this textile "nuclein". A few years later, Miescher separated nuclein into protein and nucleic acrid components. In the 1920's nucleic acids were found to be major components of chromosomes, small gene-carrying bodies in the nuclei of complex cells. Elemental analysis of nucleic acids showed the presence of phosphorus, in improver to the usual C, H, N & O. Unlike proteins, nucleic acids contained no sulfur. Complete hydrolysis of chromosomal nucleic acids gave inorganic phosphate, 2-deoxyribose (a previously unknown carbohydrate) and four dissimilar heterocyclic bases (shown in the following diagram). To reverberate the unusual sugar component, chromosomal nucleic acids are chosen deoxyribonucleic acids, abbreviated DNA. Analogous nucleic acids in which the sugar component is ribose are termed ribonucleic acids, abbreviated RNA. The acidic character of the nucleic acids was attributed to the phosphoric acid moiety.
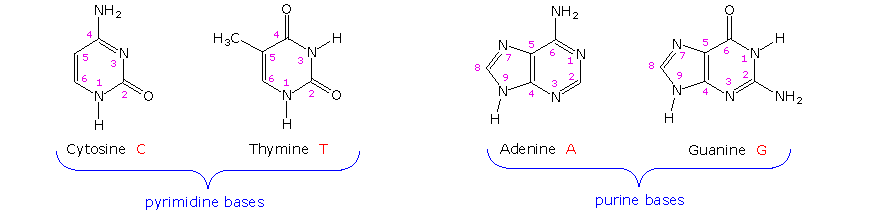
The two monocyclic bases shown here are classified equally pyrimidines, and the 2 bicyclic bases are purines. Each has at least 1 N-H site at which an organic substituent may be attached. They are all polyfunctional bases, and may exist in tautomeric forms.
Base of operations-catalyzed hydrolysis of Dna gave four nucleoside products, which proved to be N-glycosides of 2'-deoxyribose combined with the heterocyclic amines. Structures and names for these nucleosides will exist displayed above by clicking on the heterocyclic base diagram. The base components are colored green, and the sugar is black. As noted in the 2'-deoxycytidine construction on the left, the numbering of the saccharide carbons makes employ of primed numbers to distinguish them from the heterocyclic base sites. The corresponding N-glycosides of the mutual saccharide ribose are the building blocks of RNA, and are named adenosine, cytidine, guanosine and uridine (a thymidine analog missing the methyl group).
From this evidence, nucleic acids may be formulated as alternating copolymers of phosphoric acid (P) and nucleosides (Due north), every bit shown:
~ P – Northward – P – N'– P – N''– P – N'''– P – Northward ~
At first the four nucleosides, distinguished by prime number marks in this crude formula, were causeless to be present in equal amounts, resulting in a uniform construction, such every bit that of starch. However, a compound of this kind, presumably mutual to all organisms, was considered too elementary to hold the hereditary information known to reside in the chromosomes. This view was challenged in 1944, when Oswald Avery and colleagues demonstrated that bacterial DNA was likely the genetic agent that carried information from one organism to another in a process called "transformation". He concluded that "nucleic acids must exist regarded as possessing biological specificity, the chemical basis of which is equally yet undetermined." Despite this finding, many scientists continued to believe that chromosomal proteins, which differ beyond species, between individuals, and fifty-fifty within a given organism, were the locus of an organism'southward genetic information.
It should exist noted that single celled organisms like bacteria do not have a well-divers nucleus. Instead, their single chromosome is associated with specific proteins in a region chosen a "nucleoid". Nevertheless, the DNA from bacteria has the aforementioned composition and full general construction as that from multicellular organisms, including human beings.
Views about the office of Dna in inheritance inverse in the late 1940's and early on 1950'south. By conducting a careful analysis of Dna from many sources, Erwin Chargaff found its composition to be species specific. In addition, he found that the amount of adenine (A) e'er equaled the amount of thymine (T), and the amount of guanine (G) ever equaled the amount of cytosine (C), regardless of the Deoxyribonucleic acid source. As set forth in the following table, the ratio of (A+T) to (C+G) varied from 2.lxx to 0.35. The last 2 organisms are bacteria.
| Organism | Base Composition (mole %) | Base Ratios | Ratio (A+T)/(Chiliad+C) | ||||
|---|---|---|---|---|---|---|---|
| A | G | T | C | A/T | G/C | ||
| Human | 30.9 | 19.9 | 29.4 | nineteen.eight | 1.05 | one.00 | one.52 |
| Chicken | 28.8 | 20.5 | 29.ii | 21.five | 1.02 | 0.95 | 1.38 |
| Yeast | 31.three | 18.7 | 32.nine | 17.1 | 0.95 | 1.09 | one.79 |
| Clostridium | 36.9 | fourteen.0 | 36.3 | 12.eight | 1.01 | i.09 | ii.seventy |
| Sarcina | xiii.4 | 37.one | 12.4 | 37.1 | i.08 | 1.00 | 0.35 |
In a second critical report, Alfred Hershey and Martha Chase showed that when a bacterium is infected and genetically transformed by a virus, at to the lowest degree fourscore% of the viral DNA enters the bacterial cell and at to the lowest degree 80% of the viral protein remains outside. Together with the Chargaff findings this piece of work established Dna as the repository of the unique genetic characteristics of an organism.
.2. The Chemical Nature of Deoxyribonucleic acid
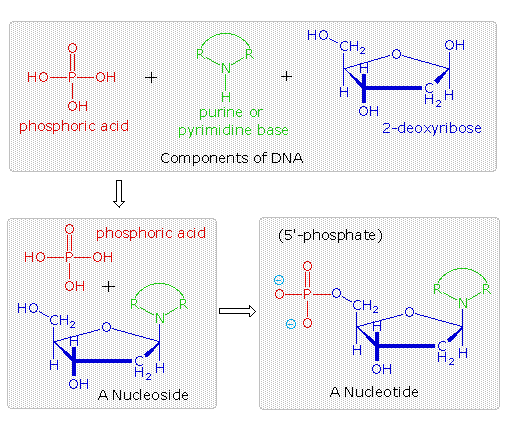
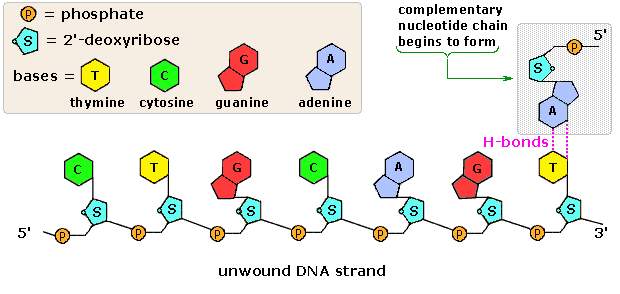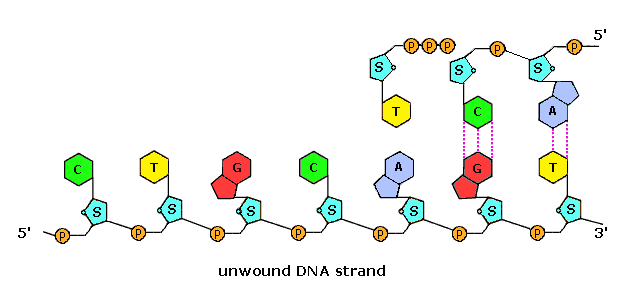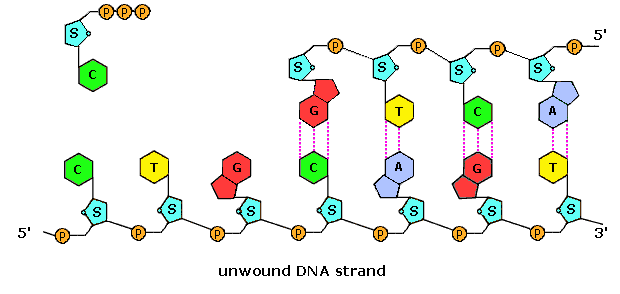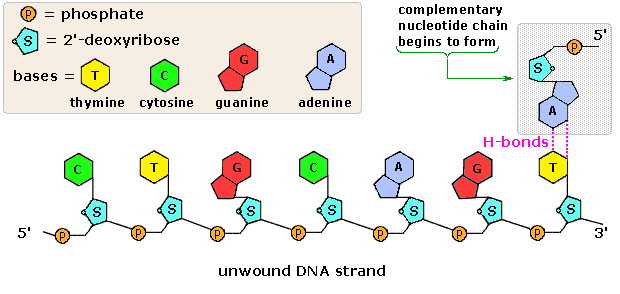
The polymeric construction of Deoxyribonucleic acid may be described in terms of monomeric units of increasing complication. In the elevation shaded box of the following illustration, the iii relatively unproblematic components mentioned before are shown. Below that on the left , formulas for phosphoric acid and a nucleoside are drawn. Condensation polymerization of these leads to the DNA formulation outlined in a higher place. Finally, a five'- monophosphate ester, chosen a nucleotide may be drawn equally a unmarried monomer unit of measurement, shown in the shaded box to the correct. Since a monophosphate ester of this kind is a stiff acid (pKa of one.0), information technology volition be fully ionized at the usual physiological pH (ca.vii.4). Names for these DNA components are given in the tabular array to the correct of the diagram. Isomeric 3'-monophospate nucleotides are also known, and both isomers are institute in cells. They may be obtained past selective hydrolysis of DNA through the activeness of nuclease enzymes. Anhydride-like di- and tri-phosphate nucleotides have been identified as of import free energy carriers in biochemical reactions, the virtually common being ATP (adenosine 5'-triphosphate).
 |
|
A complete structural representation of a segment of the Deoxyribonucleic acid polymer formed from v'-nucleotides may be viewed by clicking on the above diagram. Several important characteristics of this formula should exist noted.
• First, the remaining P-OH office is quite acidic and is completely ionized in biological systems.
• 2d, the polymer chain is structurally directed. I end (5') is different from the other (3').
• Third, although this appears to exist a relatively unproblematic polymer, the possible permutations of the 4 nucleosides in the chain get very big every bit the chain lengthens.
• Quaternary, the DNA polymer is much larger than originally believed. Molecular weights for the Deoxyribonucleic acid from multicellular organisms are commonly 109 or greater.
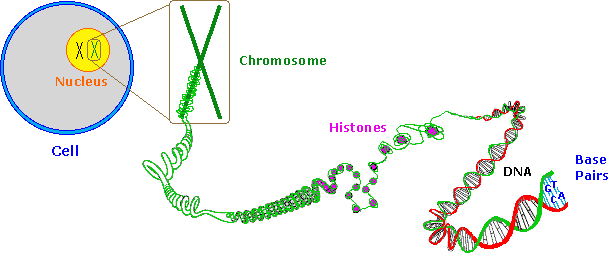
Information is stored or encoded in the DNA polymer by the pattern in which the four nucleotides are arranged. To admission this information the blueprint must be "read" in a linear way, simply as a bar lawmaking is read at a supermarket checkout. Because living organisms are extremely complex, a correspondingly large amount of information related to this complexity must be stored in the Deoxyribonucleic acid. Consequently, the Dna itself must be very big, as noted above. Even the single Deoxyribonucleic acid molecule from an E. coli bacterium is found to have roughly a million nucleotide units in a polymer strand, and would achieve a millimeter in length if stretched out. The nuclei of multicellular organisms incorporate chromosomes, which are composed of DNA combined with nuclear proteins called histones. The fruit fly has 8 chromosomes, humans have 46 and dogs 78 (annotation that the amount of DNA in a prison cell's nucleus does not correlate with the number of chromosomes). The Deoxyribonucleic acid from the smallest human chromosome is over ten times larger than E. coli DNA, and it has been estimated that the total Dna in a homo cell would extend to 2 meters in length if unraveled. Since the nucleus is just virtually 5μm in diameter, the chromosomal Dna must be packed tightly to fit in that small volume.
In addition to its function as a stable informational library, chromosomal Dna must be structured or organized in such a way that the chemical machinery of the jail cell will have easy access to that information, in order to brand important molecules such as polypeptides. Furthermore, authentic copies of the DNA lawmaking must be created equally cells split up, with the replicated Dna molecules passed on to subsequent cell generations, as well as to progeny of the organism. The nature of this DNA organization, or secondary structure, volition exist discussed in a later on section.
three. RNA, a Different Nucleic Acrid
The high molecular weight nucleic acrid, Deoxyribonucleic acid, is found importantly in the nuclei of complex cells, known equally eucaryotic cells, or in the nucleoid regions of procaryotic cells, such every bit bacteria. It is oft associated with proteins that help to pack it in a usable mode.
In contrast, a lower molecular weight, but much more abundant nucleic acid, RNA, is distributed throughout the cell, most usually in pocket-sized numerous organelles chosen ribosomes. Three kinds of RNA are identified, the largest subgroup (85 to ninety%) being ribosomal RNA, rRNA, the major component of ribosomes, together with proteins. The size of rRNA molecules varies, just is generally less than a thousandth the size of Deoxyribonucleic acid. The other forms of RNA are messenger RNA , mRNA, and transfer RNA , tRNA. Both have a more transient being and are smaller than rRNA.
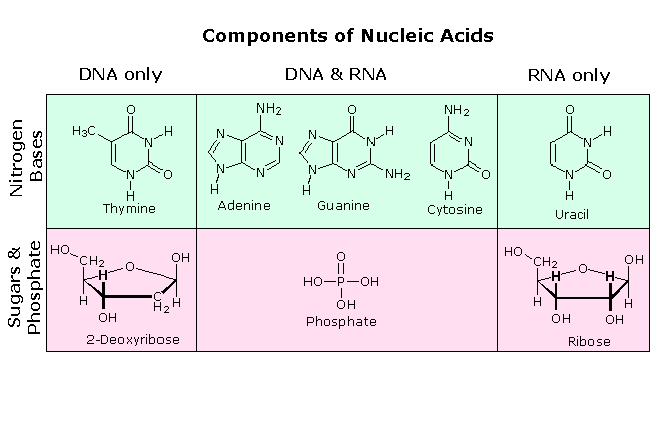
All these RNA'southward have similar constitutions, and differ from DNA in two of import respects. Equally shown in the following diagram, the sugar component of RNA is ribose, and the pyrimidine base uracil replaces the thymine base of Deoxyribonucleic acid. The RNA's play a vital role in the transfer of information (transcription) from the DNA library to the poly peptide factories called ribosomes, and in the interpretation of that information (translation) for the synthesis of specific polypeptides. These functions volition exist described afterwards.
A complete structural representation of a segment of the RNA polymer formed from 5'-nucleotides may exist viewed past clicking on the above diagram
4. The Secondary Structure of Deoxyribonucleic acid
In the early 1950's the primary structure of DNA was well established, but a firm understanding of its secondary construction was lacking. Indeed, the situation was like to that occupied past the proteins a decade before, earlier the blastoff helix and pleated canvas structures were proposed by Linus Pauling. Many researchers grappled with this problem, and information technology was by and large conceded that the tooth equivalences of base of operations pairs (A & T and C & G) discovered past Chargaff would exist an important cistron. Rosalind Franklin, working at King's College, London, obtained X-ray diffraction prove that suggested a long helical construction of uniform thickness. Francis Crick and James Watson, at Cambridge Academy, considered hydrogen bonded base pairing interactions, and arrived at a double stranded helical model that satisfied nearly of the known facts, and has been confirmed past subsequent findings.
Base Pairing
Careful examination of the purine and pyrimidine base components of the nucleotides reveals that iii of them could exist equally hydroxy pyrimidine or purine tautomers, having an aromatic heterocyclic band. Despite the added stabilization of an aromatic ring, these compounds prefer to adopt amide-similar structures. These options are shown in the following diagram, with the more than stable tautomer drawn in bluish.
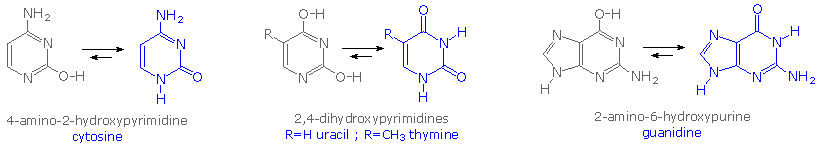
A simple model for this tautomerism is provided by 2-hydroxypyridine. As shown on the left below, a chemical compound having this structure might exist expected to have phenol-like characteristics, such as an acidic hydroxyl group. Notwithstanding, the boiling point of the bodily substance is 100º C greater than phenol and its acidity is 100 times less than expected (pKa = eleven.7). These differences agree with the 2-pyridone tautomer, the stable form of the zwitterionic internal salt. Further prove supporting this assignment volition be displayed by clicking on the diagram.
Note that this tautomerism reverses the hydrogen bonding behavior of the nitrogen and oxygen functions (the N-H grouping of the pyridone becomes a hydrogen bond donor and the carbonyl oxygen an acceptor).
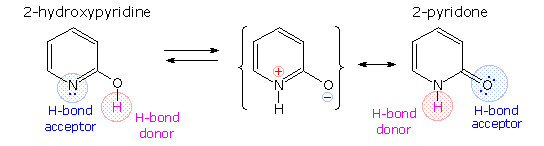
The additional evidence for the pyridone tautomer, that appears above by clicking on the diagram, consists of infrared and carbon nmr absorptions associated with and feature of the amide group. The data for ii-pyridone is given on the left. Similar data for the N-methyl derivative, which cannot tautomerize to a pyridine derivative, is presented on the right.
Once they had identified the favored base tautomers in the nucleosides, Watson and Crick were able to propose a complementary pairing, via hydrogen bonding, of guanosine (G) with cytidine (C) and adenosine (A) with thymidine (T). This pairing, which is shown in the post-obit diagram, explained Chargaff's findings beautifully, and led them to suggest a double helix structure for Dna.
Earlier viewing this double helix structure itself, information technology is instructive to examine the base pairing interactions in greater particular. The G#C association involves three hydrogen bonds (colored pink), and is therefore stronger than the two-hydrogen bond association of A#T. These base pairings might appear to be capricious, but other possibilities suffer destabilizing steric or electronic interactions. Past clicking on the diagram two such alternative couplings will exist shown. The C#T pairing on the left suffers from carbonyl dipole repulsion, as well every bit steric crowding of the oxygens. The G#A pairing on the correct is also destabilized by steric crowding (circled hydrogens).
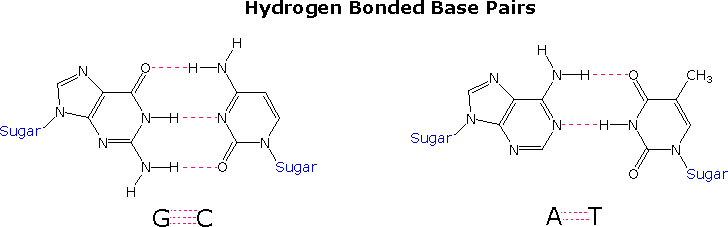
A unproblematic mnemonic device for remembering which bases are paired comes from the line construction of the capital letters used to identify the bases. A and T are made up of intersecting direct lines. In contrast, C and G are largely composed of curved lines. The RNA base uracil corresponds to thymine, since U follows T in the alphabet.
The Double Helix
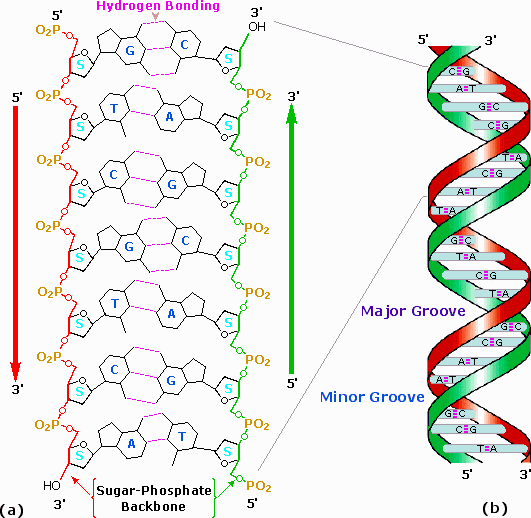
Subsequently many trials and modifications, Watson and Crick conceived an ingenious double helix model for the secondary structure of DNA. Ii strands of DNA were aligned anti-parallel to each other, i.e. with opposite 3' and 5' ends , equally shown in office a of the following diagram. Complementary primary nucleotide structures for each strand allowed intra-strand hydrogen bonding betwixt each pair of bases. These complementary strands are colored red and green in the diagram. Coiling these coupled strands then leads to a double helix structure, shown as cross-linked ribbons in office b of the diagram. The double helix is further stabilized by hydrophobic attractions and pi-stacking of the bases. A space-filling molecular model of a short segment is displayed in part c on the right.
The helix shown here has ten base pairs per turn, and rises 3.four Å in each plough. This right-handed helix is the favored conformation in aqueous systems, and has been termed the B-helix. As the Dna strands air current around each other, they leave gaps between each fix of phosphate backbones. Two alternate grooves result, a broad and deep major groove (ca. 22Å wide), and a shallow and narrow modest groove (ca. 12Å wide). Other molecules, including polypeptides, may insert into these grooves, and in then doing adjy the chemistry of DNA. Other helical structures of Dna have as well been observed, and are designated by letters (e.g. A and Z).
 | Space-Filling Molecular Model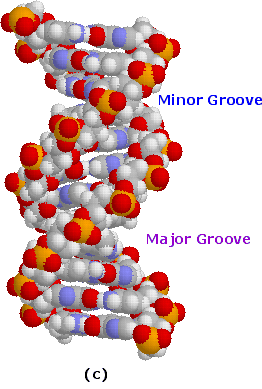 |
A model of a short DNA segment may be examined by
First-class sites, incorporating Chime and Jmol models for visualizing DNA, has been created by:
Eric Martz, Univ. Mass. Amherst. Click Here.
Frieda Reichsman, Univ. Mass. Amherst. Click Hither
1. Dna Replication
In their 1953 announcement of a double helix construction for Deoxyribonucleic acid, Watson and Crick stated, "It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic textile.". The essence of this suggestion is that, if separated, each strand of the molecule might human activity as a template on which a new complementary strand might exist assembled, leading finally to two identical Dna molecules. Indeed, replication does have identify in this manner when cells carve up, but the events leading upward to the actual synthesis of complementary Deoxyribonucleic acid strands are sufficiently complex that they will not be described in any detail.
As depicted in the following drawing, the Dna of a jail cell is tightly packed into chromosomes. Kickoff, the DNA is wrapped around pocket-size proteins called histones (colored pink below). These bead-similar structures are then further organized and folded into chromatin aggregates that make upward the chromosomes. An overall packing efficiency of vii,000 or more is thus achieved. Clearly a sequence of unfolding events must take identify earlier the data encoded in the DNA can be used or replicated.
In one case the double stranded Dna is exposed, a group of enzymes act to achieve its replication. These are described briefly here:
Topoisomerase: This enzyme initiates unwinding of the double helix by cutting one of the strands.
Helicase: This enzyme assists the unwinding. Note that many hydrogen bonds must be broken if the strands are to be separated..
SSB: A single-strand binding-poly peptide stabilizes the separated strands, and prevents them from recombining, so that the polymerization chemical science can function on the individual strands.
Deoxyribonucleic acid Polymerase: This family of enzymes link together nucleotide triphosphate monomers as they hydrogen bond to complementary bases. These enzymes also bank check for errors (roughly ten per billion), and brand corrections.
Ligase: Small unattached DNA segments on a strand are united past this enzyme.
Polymerization of nucleotides takes place past the phosphorylation reaction described by the following equation.
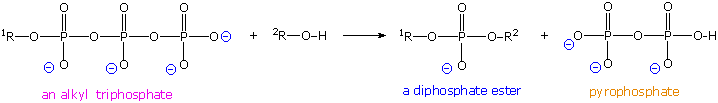
Di- and triphosphate esters accept anhydride-like structures and are consequently reactive phosphorylating reagents, just as carboxylic anhydrides are acylating reagents. Since the pyrophosphate anion is a ameliorate leaving grouping than phosphate, triphosphates are more powerful phosphorylating agents than are diphosphates. Formulas for the corresponding v'-derivatives of adenosine will be displayed by Clicking Hither, and similar derivatives exist for the other three common nucleosides. The Dna polymerization process that builds the complementary strands in replication, could in principle accept place in two ways. Referring to the full general equation above, R1 could represent the adjacent nucleotide unit to be attached to the growing DNA strand, with R2 beingness this strand. Alternatively, these assignments could be reversed. In practice, the former proves to be the best arrangement. Since triphosphates are very reactive, the lifetime of such derivatives in an aqueous environment is relatively brusque. Yet, such derivatives of the individual nucleosides are repeatedly synthesized by the jail cell for a multifariousness of purposes, providing a steady supply of these reagents. In contrast, the growing Deoxyribonucleic acid segment must maintain its functionality over the unabridged replication process, and can not afford to be changed by a spontaneous hydrolysis event. As a result, these chemic properties are best accommodated by a polymerization procedure that proceeds at the three'-end of the growing strand past v'-phosphorylation involving a nucleotide triphosphate. This process is illustrated by the post-obit animation, which may exist activated by clicking on the diagram or reloading the page.
The polymerization mechanism described hither is abiding. It always extends the developing DNA segment toward the three'-cease (i.e. when a nucleotide triphosphate attaches to the free 3'-hydroxyl grouping of the strand, a new iii'-hydroxyl is generated). There is sometimes confusion on this point, because the original Deoxyribonucleic acid strand that serves every bit a template is read from the three'-end toward the 5'-finish, and authors may not be completely clear as to which terminology is used.
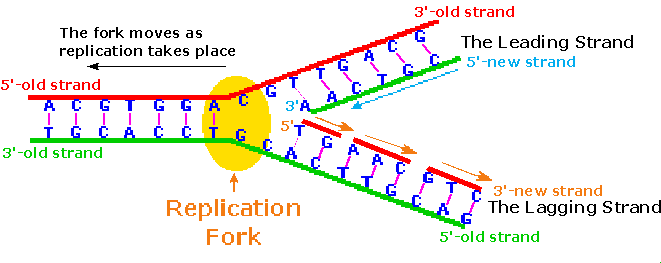
Considering of the directional need of the polymerization, one of the Deoxyribonucleic acid strands is easily replicated in a continuous mode, whereas the other strand tin can only be replicated in brusque segmental pieces. This is illustrated in the following diagram. Separation of a portion of the double helix takes identify at a site called the replication fork. Equally replication of the divide strands occurs, the replication fork moves abroad (to the left in the diagram), unwinding boosted lengths of DNA. Since the fork in the diagram is moving toward the 5'-end of the red-colored strand, replication of this strand may take place in a continuous fashion (building the new green strand in a 5' to iii' management). This continuously formed new strand is called the leading strand. In contrast, the replication fork moves toward the 3'-stop of the original green strand, preventing continuous polymerization of a complementary new red strand. Short segments of complementary DNA, called Okazaki fragments, are produced, and these are linked together later past the enzyme ligase. This new DNA strand is called the lagging strand.
When you consider that a human being cell has roughly x9 base pairs in its DNA, and may divide into identical daughter cells in 14 to 24 hours, the efficiency of Dna replication must be extraordinary. The procedure described above volition replicate about 50 nucleotides per 2nd, so there must exist many thousand such replication sites in action during cell sectionalization. A given length of double stranded DNA may undergo strand unwinding at numerous sites in response to promoter actions. The unraveled "chimera" of single stranded Dna has two replication forks, so assembly of new complementary strands may go along in ii directions. The polymerizations associated with several such bubbles fuse together to achieve full replication of the entire Dna double helix. A cartoon illustrating these concerted replications will appear by clicking on the above diagram. Note that the events shown go along from top to bottom in the diagram.
two. Repair of Dna Damage and Replication Errors
One of the benefits of the double stranded DNA structure is that it lends itself to repair, when structural damage or replication errors occur. Several kinds of chemical alter may crusade harm to Dna:
• Spontaneous hydrolysis of a nucleoside removes the heterocyclic base component.
• Spontaneous hydrolysis of cytosine changes information technology to a uracil.
• Diverse toxic metabolites may oxidize or methylate heterocyclic base components.
• Ultraviolet light may dimerize adjacent cytosine or thymine bases.
All these transformations disrupt base pairing at the site of the change, and this produces a structural deformation in the double helix.. Inspection-repair enzymes find such deformations, and use the undamaged nucleotide at that site equally a template for replacing the damaged unit. These repairs reduce errors in DNA structure from nigh 1 in ten million to ane per trillion.
RNA and Protein Synthesis
The genetic information stored in Deoxyribonucleic acid molecules is used equally a blueprint for making proteins. Why proteins? Because these macromolecules take diverse primary, secondary and tertiary structures that equip them to carry out the numerous functions necessary to maintain a living organism. As noted in the protein affiliate, these functions include:
• Structural integrity (hair, horn, center lenses etc.).
• Molecular recognition and signaling (antibodies and hormones).
• Catalysis of reactions (enzymes)..
• Molecular ship (hemoglobin transports oxygen).
• Motility (pumps and motors).
The critical importance of proteins in life processes is demonstrated past numerous genetic diseases, in which small modifications in primary structure produce debilitating and often disastrous consequences. Such genetic diseases include Tay-Sachs, phenylketonuria (PKU), sickel cell anemia, achondroplasia, and Parkinson disease. The unavoidable conclusion is that proteins are of central importance in living cells, and that proteins must therefore exist continuously prepared with high structural fidelity past appropriate cellular chemistry.
Early on geneticists identified genes every bit hereditary units that determined the appearance and / or function of an organism (i.e. its phenotype). We now define genes as sequences of Dna that occupy specific locations on a chromosome. The original proposal that each factor controlled the formation of a single enzyme has since been modified as: one cistron = one polypeptide. The intriguing question of how the information encoded in Deoxyribonucleic acid is converted to the actual construction of a specific polypeptide has been the subject of numerous studies, which have created the modern field of Molecular Biology.
1. The Central Dogma and Transcription
Francis Crick proposed that information flows from Deoxyribonucleic acid to RNA in a procedure called transcription, and is so used to synthesize polypeptides by a procedure chosen translation. Transcription takes place in a manner similar to DNA replication. A characteristic sequence of nucleotides marks the beginning of a factor on the Deoxyribonucleic acid strand, and this region binds to a promoter protein that initiates RNA synthesis. The double stranded structure unwinds at the promoter site., and one of the strands serves as a template for RNA formation, as depicted in the post-obit diagram. The RNA molecule thus formed is unmarried stranded, and serves to acquit information from DNA to the protein synthesis machinery called ribosomes. These RNA molecules are therefore called messenger-RNA (mRNA).
To summarize: a gene is a stretch of Dna that contains a blueprint for the amino acid sequence of a poly peptide. In order to actually make this protein, the relevant Dna segment is first copied into messenger-RNA. The cell then synthesizes the poly peptide, using the mRNA as a template.
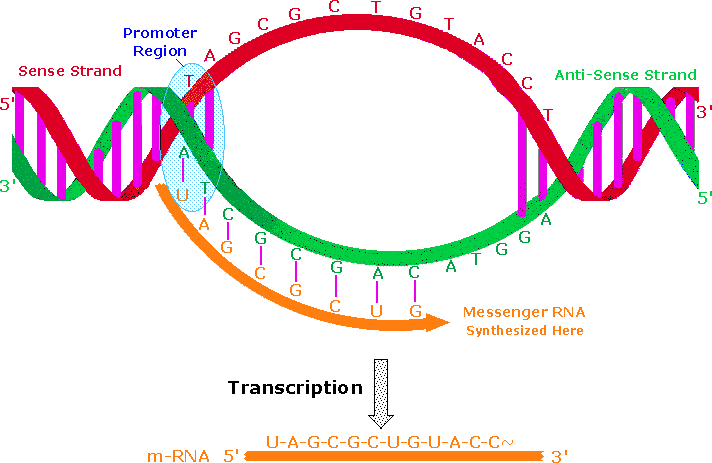
An important distinction must be fabricated hither. One of the Deoxyribonucleic acid strands in the double helix holds the genetic data used for protein synthesis. This is called the sense strand, or information strand (colored carmine above). The complementary strand that binds to the sense strand is called the anti-sense strand (colored light-green), and it serves as a template for generating a mRNA molecule that delivers a copy of the sense strand information to a ribosome. The promoter poly peptide binds to a specific nucleotide sequence that identifies the sense strand, relative to the anti-sense strand. RNA synthesis is and then initiated in the 3' direction, as nucleotide triphosphates bind to complementary bases on the template strand, and are joined past phosphate diester linkages. An animation of this procedure for Dna replication was presented earlier. A characteristic "stop sequence" of nucleotides terminates the RNA synthesis. The messenger molecule (colored orangish in a higher place) is released into the cytoplasm to find a ribosome, and the Dna and so rewinds to its double helix construction.
In eucaryotic cells the initially transcribed m-RNA molecule is usually modified and shortened by an "editing" procedure that removes irrelevant material. The DNA of such organisms is oftentimes thousands of times larger and more complex than that composing the single chromosome of a procaryotic bacterial prison cell. This difference is due in part to repetitive nucleotide sequences (ca. 25% in the human being genome). Furthermore, over 95% of human Deoxyribonucleic acid is found in intervening sequences that carve up genes and parts of genes. The informational Dna segments that make up genes are called exons, and the noncoding segments are called introns. Before the mRNA molecule leaves the nucleus, the nonsense bases that make upward the introns are cut out, and the informationally useful exons are joined together in a pace known every bit RNA splicing. In this manner shorter mRNA molecules carrying the design for a specific protein are sent on their way to the ribosome factories.
The Central Dogma of molecular biology, which at first was formulated as a simple linear progression of information from DNA to RNA to Poly peptide, is summarized in the following analogy. The replication procedure on the left consists of passing information from a parent Dna molecule to daughter molecules. The middle transcription procedure copies this information to a mRNA molecule. Finally, this data is used by the chemical machinery of the ribosome to brand polypeptides.
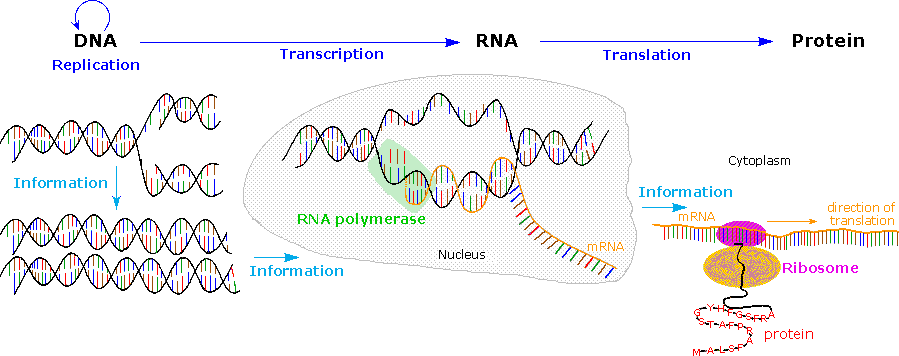
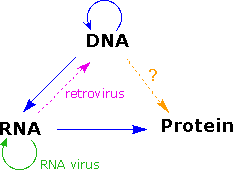
As more has been learned about these relationships, the central dogma has been refined to the representation displayed on the right. The dark blue arrows evidence the full general, well demonstrated, data transfers noted above. It is now known that an RNA-dependent DNA polymerase enzyme, known equally a reverse transcriptase, is able to transcribe a unmarried-stranded RNA sequence into double-stranded DNA (magenta pointer). Such enzymes are found in all cells and are an essential component of retroviruses (e.g. HIV), which require RNA replication of their genomes (green arrow). Direct translation of DNA information into poly peptide synthesis (orange pointer) has non however been observed in a living organism. Finally, proteins announced to exist an advisory dead stop, and do not provide a structural pattern for either RNA or DNA.
In the following section the concluding key relationship, that of structural information translation from mRNA to protein, will exist described
2. Translation
Translation is a more complex procedure than transcription. This would, of course, exist expected. After all, the coded letters produced by the German Enigma machine could be copied easily, but required a considerable decoding effort earlier they could be read with understanding. In a like sense, Dna replication is simply a complementary base pairing exercise, but the translation of the four letter (bases) alphabet code of RNA to the xx alphabetic character (amino acids) alphabet of protein literature is far from piddling. Clearly, there could not be a direct 1-to-i correlation of bases to amino acids, so the nucleotide messages must form short words or codons that ascertain specific amino acids. Many questions pertaining to this genetic code were posed in the late 1950'southward:
• How many RNA nucleotide bases designate a specific amino acid?
If carve up groups of nucleotides, called codons, serve this purpose, at least 3 are needed. There are four3 = 64 different nucleotide triplets, compared with fourii = 16 possible pairs.
• Are the codons linked separately or do they overlap?
Sequentially joined triplet codons volition effect in a nucleotide concatenation three times longer than the protein information technology describes. If overlapping codons are used then fewer total nucleotides would be required.
• If triplet segments of mRNA designate specific amino acids in the poly peptide, how are the codons identified?
For the sequence ~CUAGGU~ are the codons CUA & GGU or ~C, UAG & GU~ or ~CU, AGG & U~?
• Are all the codon words the same size?
In Morse code the most widely used letters are shorter than less common letters. Perhaps nature employs a similar scheme.
Physicists and mathematicians, equally well as chemists and microbiologists all contributed to unravelling the genetic code. Although before proposals assumed efficient relationships that correlated the nucleotide codons uniquely with the xx fundamental amino acids, it is now credible that there is considerable back-up in the code as it now operates. Furthermore, the code consists exclusively of non-overlapping triplet codons.
Clever experiments provided some of the earliest breaks in deciphering the genetic code. Marshall Nirenberg found that RNA from many unlike organisms could initiate specific poly peptide synthesis when combined with broken Due east.coli cells (the enzymes remain agile). A constructed polyuridine RNA induced synthesis of poly-phenylalanine, and then the UUU codon designated phenylalanine. Too an alternating ~CACA~ RNA led to synthesis of a ~His-Thr-His-Thr~ polypeptide.
The following tabular array presents the present solar day estimation of the genetic code. Note that this is the RNA alphabet, and an equivalent Deoxyribonucleic acid codon tabular array would have all the U nucleotides replaced past T. Methionine and tryptophan are uniquely represented past a single codon. At the other extreme, leucine is represented by 8 codons. The average redundancy for the xx amino acids is near iii. Besides, at that place are three stop codons that terminate polypeptide synthesis.
| Second Position | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U | C | A | Thousand | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| F P | U |
|
|
|
|
| T P | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| C |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| G |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
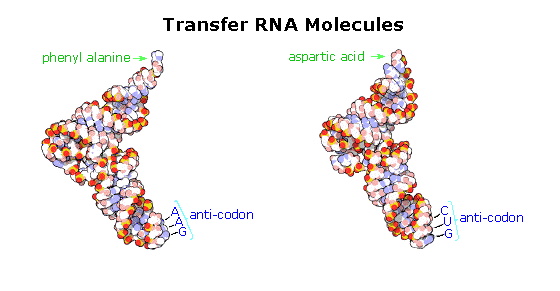
The translation process is fundamentally straightforward. The mRNA strand bearing the transcribed code for synthesis of a protein interacts with relatively small-scale RNA molecules (about lxx-nucleotides) to which individual amino acids have been attached by an ester bond at the iii'-end. These transfer RNA'due south (tRNA) take distinctive three-dimensional structures consisting of loops of single-stranded RNA connected past double stranded segments. This cloverleaf secondary structure is further wrapped into an "L-shaped" assembly, having the amino acid at the end of one arm, and a characteristic anti-codon region at the other end. The anti-codon consists of a nucleotide triplet that is the complement of the amino acid's codon(s). Models of two such tRNA molecules are shown to the correct. When read from the tiptop to the bottom, the anti-codons depicted hither should complement a codon in the previous table.
Cloverleaf cartoons of three other tRNA molecules will be shown on the right by clicking on the diagram.
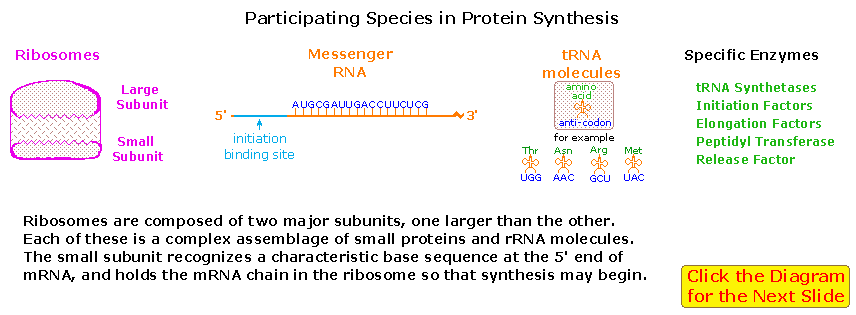
A cell's protein synthesis takes place in organelles called ribosomes. Ribosomes are complex structures fabricated up of two distinct and separable subunits (one nigh twice the size of the other). Each subunit is composed of one or two RNA molecules (60-70%) associated with 20 to xl pocket-size proteins (30-40%). The ribosome accepts a mRNA molecule, binding initially to a characteristic nucleotide sequence at the 5'-finish (colored light blue in the following diagram). This unique bounden assures that polypeptide synthesis starts at the right codon. A tRNA molecule with the advisable anti-codon then attaches at the starting point and this is followed by a serial of adjacent tRNA attachments, peptide bond formation and shifts of the ribosome along the mRNA chain to expose new codons to the ribosomal chemistry.
The post-obit diagram is designed as a slide bear witness illustrating these steps. The issue is synthesis of a polypeptide chain corresponding to the mRNA blueprint. A "stop codon" at a designated position on the mRNA terminates the synthesis by introduction of a "Release Gene".
To visit an informative Tour of the Ribosome site, created by Wayne Decatur, Univ. Mass. Amherst Click Here.
3. Post-translational Modification
One time a peptide or protein has been synthesized and released from the ribosome information technology often undergoes further chemical transformation. This mail-translational modification may involve the attachment of other moieties such as acyl groups, alkyl groups, phosphates, sulfates, lipids and carbohydrates. Functional changes such equally dehydration, amidation, hydrolysis and oxidation (e.one thousand. disulfide bond germination) are also mutual. In this manner the limited array of 20 amino acids designated by the codons may be expanded in a variety of means to enable proper operation of the resulting protein. Since these post-translational reactions are generally catalyzed by enzymes, information technology may be said: "Virtually every molecule in a cell is made past the ribosome or by enzymes made by the ribosome."
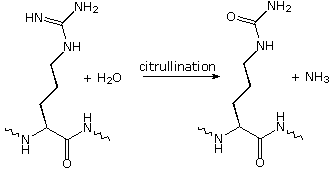
Modifications, like phosphorylation and citrullination, are part of common mechanisms for controlling the behavior of a protein. Equally shown on the left beneath, citrullination is the mail service-translational modification of the amino acid arginine into the amino acrid citrulline. Arginine is positively charged at a neutral pH, whereas citrulline is uncharged, so this change increases the hydrophobicity of a protein. Phosphorylation of serine, threonine or tyrosine residues renders them more hydrophilic, but such changes are usually transient, serving to regulate the biological activity of the protein. Other important functional changes include iodination of tyrosine residues in the peptide thyroglobulin by action of the enzyme thyroperoxidase. The monoiodotyrosine and diiodotyrosine formed in this manner are then linked to class the thyroid hormones T3 and T4, shown on the right below.
 | 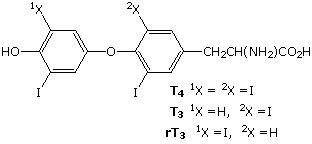 |
|---|
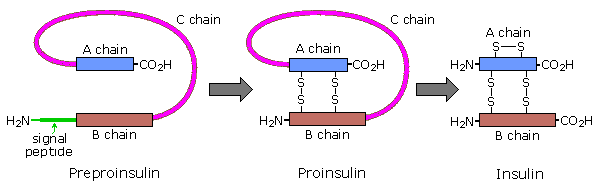
Amino acids may be enzymatically removed from the amino stop of the poly peptide. Because the "start" codon on mRNA codes for the amino acrid methionine, this amino acid is commonly removed from the resulting protein during post-translational modification. Peptide chains may too be cut in the middle to grade shorter strands. Thus, insulin is initially synthesized as a 105 residue preprotein. The 24-amino acid signal peptide is removed, yielding a proinsulin peptide. This folds and forms disulfide bonds between cysteines 7 and 67 and betwixt 19 and 80. Such dimeric cysteines, joined by a disulfide bail, are named cystine. A protease and then cleaves the peptide at arg31 and arg60, with loss of the 32-threescore sequence (concatenation C). Removal of arg31 yields mature insulin, with the A and B chains held together past disulfide bonds and a third cystine moiety in chain A. The following cartoon illustrates this chain of events.
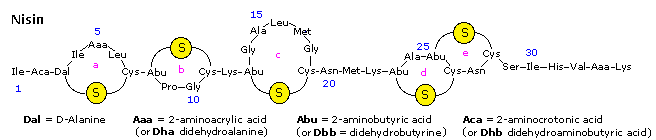
Nisin is a polypeptide (34 amino acids) made by the bacterium Lactococcus lactis. Nisin kills gram positive leaner by binding to their membranes and targeting lipid Two, an essential precursor of prison cell wall synthesis. Such antimicrobial peptides are a growing family of compounds which accept received the name lantibiotics due to the presence of lanthionine, a nonproteinogenic amino acrid with the chemic formula HO2C-CH(NH2)-CHtwo-Southward-CH2-CH(NH2)-CO2H. Lanthionine is composed of 2 alanine residues that are crosslinked on their β-carbon atoms by a thioether linkage (i.e. information technology is the monosulfide analog of the disulfide cystine). Lantibiotics are unique in that they are ribosomally synthesized equally prepeptides, followed past post-translational processing of a number of amino acids (e.g. serine, threonine and cysteine) into dehydro residues and thioether crossbridges. Nisin is the only bacteriocin that is accepted as a food preservative. Several nisin subtypes that differ in amino acrid composition and biological action are known. A typical structure is drawn beneath, and a Jmol model will be presented by clicking on the diagram.
The bacterial jail cell wall is a cross-linked glycan polymer that surrounds bacterial cells, dictates their cell shape, and prevents them from breaking due to environmental changes in osmotic pressure. This wall consists mainly of peptidoglycan or murein, a three-dimensional polymer of sugars and amino acids located on the exterior of the cytoplasmic membrane. 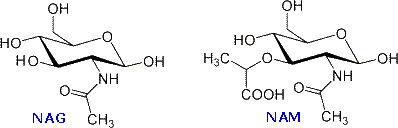 The monomer units are composed of two amino sugars, N-acetylglucosamine (NAG) and North-acetylmuramic acrid (NAM), shown on the right. Transglycosidase enzymes join these units by glycoside bonds, and they are further interlinked to each other via peptide cross-links between the pentapeptide moieties that are attached to the NAM residues. Peptidoglycan subunits are assembled on the cytoplasmic side of the bacterial membrane from a polyisoprenoid ballast. Lipid Ii, a membrane-anchored jail cell-wall precursor that is essential for bacterial cell-wall biosynthesis, is one of the key components in the synthesis of peptidoglycan. Peptidoglycan synthesis via polymerization of Lipid Two is illustrated in the following diagram. Cross-linking of the peptide side chains is then effected by transpeptidase enzymes. A model of Lipid Ii complexed with nisin may be examined every bit office of the previous Jmol display.
The monomer units are composed of two amino sugars, N-acetylglucosamine (NAG) and North-acetylmuramic acrid (NAM), shown on the right. Transglycosidase enzymes join these units by glycoside bonds, and they are further interlinked to each other via peptide cross-links between the pentapeptide moieties that are attached to the NAM residues. Peptidoglycan subunits are assembled on the cytoplasmic side of the bacterial membrane from a polyisoprenoid ballast. Lipid Ii, a membrane-anchored jail cell-wall precursor that is essential for bacterial cell-wall biosynthesis, is one of the key components in the synthesis of peptidoglycan. Peptidoglycan synthesis via polymerization of Lipid Two is illustrated in the following diagram. Cross-linking of the peptide side chains is then effected by transpeptidase enzymes. A model of Lipid Ii complexed with nisin may be examined every bit office of the previous Jmol display.
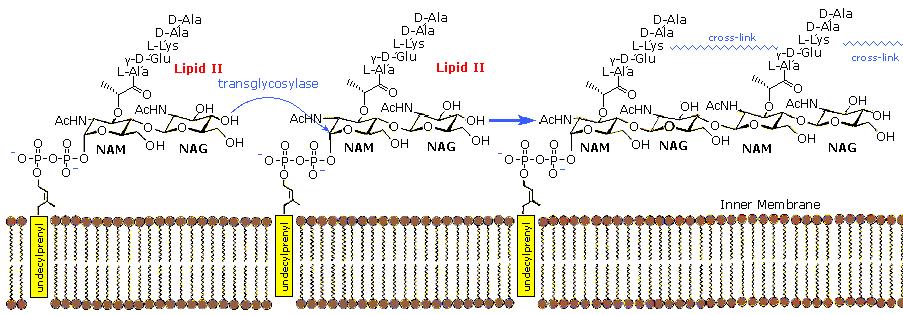
In guild for leaner to divide past binary fission and increase their size following division, links in the peptidoglycan must be broken, new peptidoglycan monomers must exist inserted, and the peptide cross links must be resealed. Transglycosidase enzymes catalyze the germination of glycosidic bonds between the NAM and NAG of the peptidoglycan monomers and the NAG and NAM of the existing peptidoglycan. Finally, transpeptidase enzymes reform the peptide cross-links between the rows and layers of peptidoglycan making the wall stiff. Many antibiotic drugs, including penicillin, target the chemistry of prison cell wall formation. The effectiveness of choosing Lipid Two for an antibacterial strategy is highlighted past the fact that it is the target for at least four different classes of antibiotic, including the clinically of import glycopeptide antibiotic vancomycin. The growing problem of bacterial resistance to many electric current drugs, including vancomycin, has led to increasing involvement in the therapeutic potential of other classes of chemical compound that target Lipid Ii. Lantibiotics such as nisin are part of this interest.
For a speculative word of why nature selected the components and functional groups found in the nucleic acids Click Here.
| Return to Tabular array of Contents |
|---|
This page is the property of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in capacity building in chemic education. 05/05/2013
![]()
![]()
Analysis of Structural Similarities and Differences between Dna and RNA
1. Background
We know that living organisms accept the power to reproduce and to laissez passer many of their characteristics on to their offspring. From this we may infer that all organisms have genetic substances and an associated chemistry that enable inheritance to occur. Information technology is instructive to consider the essential requirements such genetic materials must fullfill.
Information
Biologically useful information, especially instructions for protein synthesis, must be incorporated in the fabric.
Stability
The inherited information must be stable (unchanged) over the lifetime of the organism if authentic copies are to be conveyed to the offspring. Infrequent changes may take place (see mutability).
Reproduction
A method of faithfully replicating the information encoded in the material, and transmitting this copy to the offspring must exist.
Mutability
Despite the inherent stability noted above, the material must exist capable of incorporating stable structural change, and passing this change on to succeeding generations.
Since this genetic substance has been identified as the nucleic acids Deoxyribonucleic acid and RNA, it is instructive to examine the manner in which these polymers satisfy the above requirements.
ii. Information Storage
The complexity of life suggests that even uncomplicated organisms will require very big inheritance libraries. Although the 4 nucleotides that make up of Dna might appear to be too simple for this job, the enormous size of the polymer and the permutations of the monomers within the concatenation come across the claiming easily. After all, the words and graphics in this document are all presented to the calculator as combinations of only 2 characters, zeros and ones (the binary number system). DNA has iv letters in its alphabet (A, C, Grand & T), so the number of words that tin exist formed increase exponentially with the number of letters per word. Thus, in that location are 42 or sixteen ii letter words, and 43 or 64 three alphabetic character words.
Assuring the stability of information encoded by the DNA alphabet presents a serious challenge. If the letters of this alphabet are to be strung together in a specific mode on the polymer chain, chemical reactions for attaching (and removing) them must be bachelor. Simple carboxylic ester or amide links might appear suitable for this purpose (note step-growth polymerization), but these are used in lipids and polypeptides, so a separate enzymatic machinery would exist needed to proceed the data processing operations apart from other molecular transformations.
The overall stability of such covalent links presents a more serious problem. Under physiological weather (aqueous, pH near 7.4 & 27 to 37º C) esters are slowly hydrolyzed. Amides are more than stable, but even a hydrolytic cleavage of 1 bond per hour would be devastating to a polymer having tens of thousands to millions such links. Furthermore, short difunctional linking groups, such every bit carbonates, oxylates and malonates show enhanced reactivity, and their parent acids are unstable or toxic.
| Ester | Rate of Hydrolysis | Relative Charge per unit |
|---|---|---|
| Ethyl Acetate CH3CO2C2Hv | one.0*10-2 | 5*106 |
| Trimethyl Phosphate (CH3O)3PO | three.iv*10-four | 2*x5 |
| Dimethyl Phosphate (CH3O)twoPO2 (-) | two.0*10-9 | 1.0 |
Phosphate is an ubiquitous inorganic nutrient. Mono, di and triesters of the respective acrid (phosphoric acid) are all known. Because of their acidity (pKa ≈ 2), the mono and diesters are negatively charged at physiological pH, rendering them less susceptible to nucleophilic attack. The influence of negative accuse on the charge per unit of nucleophilic hydrolysis of some representative esters is shown in the table on the correct. Clearly, a polymer in which monomer units are joined by negatively charged diphosphate ester links should exist essentially more stable than 1 composed of carboxylate ester bonds. The negative charge found on all biological phosphate derivatives serves other purposes as well.
• The diphosphate ester links that join the nucleotides units of Deoxyribonucleic acid are formed by phosphorylation reactions involving nucleotide triphosphate reagents. These reagents are the phosphoric acid analogs of carboxylic acid anhydrides, a functional group that would non survive the aqueous environment of a jail cell. The high density of negative accuse on the triphosphate role not just solubilizes the organic moiety to which information technology is attached, but also reduces the rate at which it is hydrolyzed.
• Living cells must conserve and employ their chemical reagents within a volume divers and enclosed by a membrane barrier. These lipid bilayer membranes have hydrophobic interiors, which resist the passage of ions. Indeed, special trans-membrane structures called ion channels be so that controlled ion transport across a membrane may accept place. Small neutral organic molecules, such as adenosine, cytidine and guanosine, may pass through lipid membranes, albeit at a reduced rate, simply their mono, di and triphosphate derivatives are more tightly sequestered in the cell.
iii. Why is two'-Deoxyribose the Sugar Moiety in DNA?
Common perhydroxylated sugars, such as glucose and ribose, are formed in nature as products of the reductive condensation of carbon dioxide we call photosynthesis. The formation of deoxysugars requires boosted biological reduction steps, so it is reasonable to speculate why Dna makes use of the less common 2'-deoxyribose, when ribose itself serves well for RNA. At least two bug associated with the extra hydroxyl group in ribose may be noted. First, the boosted bulk and hydrogen bonding character of the 2'-OH interfere with a uniform double helix structure, preventing the efficient packing of such a molecule in the chromosome. 2d, RNA undergoes spontaneous hydrolytic cleavage about i hundred times faster than Deoxyribonucleic acid. This is believed due to intramolecular attack of the 2'-hydroxyl function on the neighboring phosphate diester, yielding a 2',3'-circadian phosphate. If stability over the lifetime of an organism is an essential characteristic of a factor, then nature'south selection of ii'-deoxyribose for Deoxyribonucleic acid makes sense. The following diagram illustrates the intramolecular cleavage reaction in a strand of RNA.
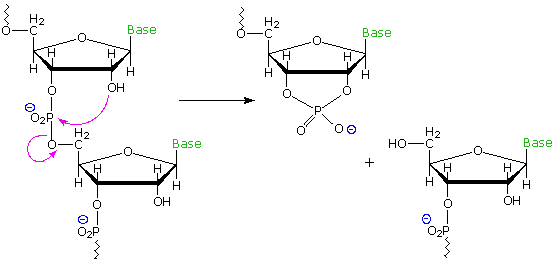
Structural stability is non a serious challenge for RNA. The transcripted data carried by mRNA must be secure for merely a few hours, as information technology is transported to a ribosome. Once in the ribosome it is surrounded by structural and enzymatic segments that immediately comprise its codons for protein synthesis. The tRNA molecules that bear amino acids to the ribosome are similarly short lived, and are in fact continuously recycled by the cellular chemistry.
4. The Thymine vs. Uracil Event
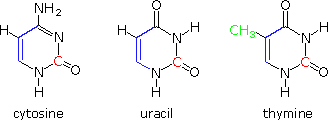 Structural formulas for the three pyrimidine bases, cytosine, thymine and uracil are shown on the correct. The carbon atoms that are part of these compounds may be categorized equally follows. All of these compounds are apparently put together from a iii-carbon malonate-like precursor (bluish colored bonds) and a single loftier oxidation country carbon species (colored cherry). Such biosynthetic intermediates are well established. Thymine is unique in having an additional carbon, the green methyl grouping. Biosynthesis of this chemical compound must involve additional steps, thus adding constructional complication to the Deoxyribonucleic acid molecules in which it replaces uracil.
Structural formulas for the three pyrimidine bases, cytosine, thymine and uracil are shown on the correct. The carbon atoms that are part of these compounds may be categorized equally follows. All of these compounds are apparently put together from a iii-carbon malonate-like precursor (bluish colored bonds) and a single loftier oxidation country carbon species (colored cherry). Such biosynthetic intermediates are well established. Thymine is unique in having an additional carbon, the green methyl grouping. Biosynthesis of this chemical compound must involve additional steps, thus adding constructional complication to the Deoxyribonucleic acid molecules in which it replaces uracil.
The reason for the substitution of thymine for uracil in Dna may exist associated with the repair mechanisms past which the prison cell corrects damage to its DNA. One source of error in the code is the slow hydrolysis of heterocyclic enamines, such as cytosine and guanine, to their respective lactams. This changes the construction of the base, and disrupts base pairing in a manner that tin be identified and and then repaired. However, the hydrolysis product from cytosine is uracil, and this mismatched species must somehow exist distinguished from the uracil-like base that belongs in the Dna. The actress methyl grouping serves this office nicely.
For a more consummate give-and-take of some of the issues touched on here run across an commodity titled "Why Nature Chose Phosphates",
authored by F. H .Westheimer, which appeared in the March sixth, 1987 outcome of Science.
| Return to Tabular array of Contents |
|---|
This folio is the property of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in chapters building in chemic didactics. 05/05/2013
![]()
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/nucacids.htm
0 Response to "How Can You Remember the Sugar Sticks to Both Nucleotide Parts"
Post a Comment